

Lithium is sometimes used as Lithium oxide (Li 2O) in special glasses and ceramics, like the glass used in the 200-inch telescope at Mt. Lithium metal is made into alloys with aluminium, magnesium, cadmium, and copper to improving their strength and making them lighter.Ī Magnesium-Lithium alloy is used for Armour Plating, and Aluminium-lithium alloys are used in bicycle frames, high-speed trains, and to make high performance Aircraft parts.
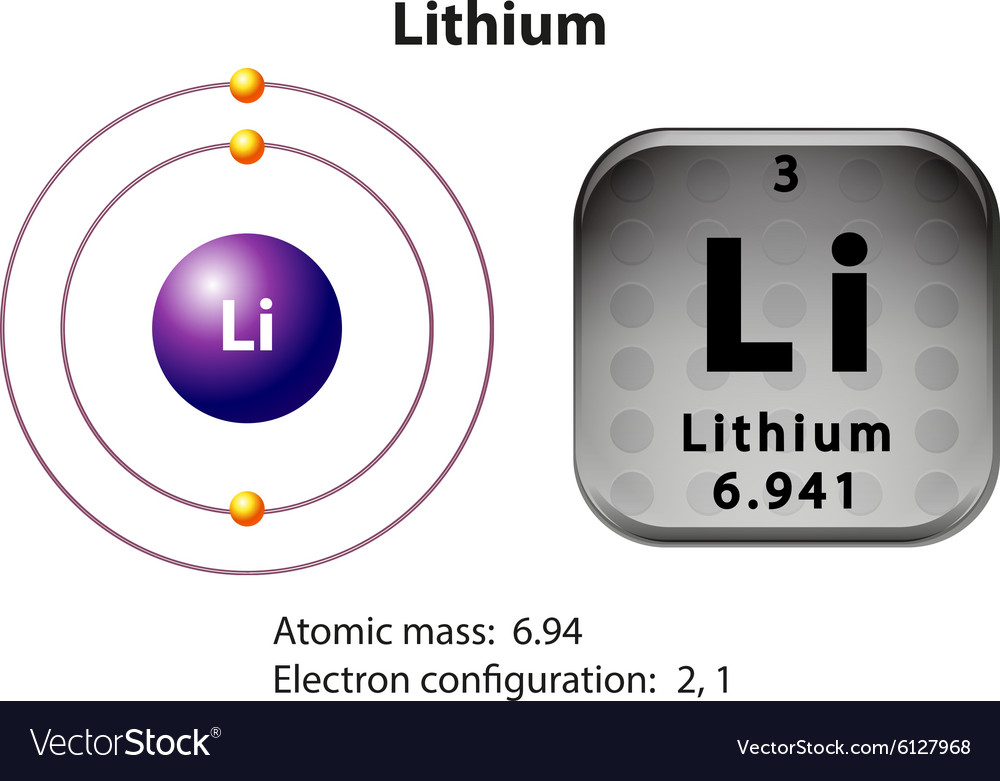
The metal is Largely uses in Rechargeable batteries for Laptops, Mobile phones, Digital camera, Electric vehicles and many more electronic gadgets and also in non-Rechargeable batteries for many things like Heart Pacemaker, Toys, Remotes. The production of lithium metal and its compounds has increased greatly. Lithium Historyĭiscovery: Johan August Arfwedson (1817), at Stockholm (Sweden)įirst Isolation: William Thomas Brande (1821) Lithium Usesīecause of its highest specific heat, Lithium is used in heat transfer applications. This reaction produces the tritium on the spot. The production of Tritium (Radioactive form of hydrogen) from Lithium Deuteride (LiD). Lithium reacts with hydrogen, and forming Lithium hydride (LiH).Ģ Li (s) + H 2 (g) → 2 LiH (s) Reaction with Neutrons Lithium Dissolves readily in dilute sulphuric acid (H 2SO 4), and forming Lithium (Li) ions and hydrogen gas (H 2).Ģ Li (s) + H 2SO 4 (aq) → 2 Li + (aq) + SO 4 2- (aq) + H 2 (g) Reaction with Hydrogen The metal reacts with the halogens, and forming the corresponding lithium halides.Ģ Li (s) + F 2 (g) → 2 LiF (s) (Lithium fluoride)Ģ Li (s) + Cl 2 (g) → 2 LiCl (s) (Lithium Chloride)Ģ Li (s) + Cl 2 (g) → 2 LiBr (s) (Lithium Bromide)Ģ Li (s) + I 2 (g) → 2 LiI (s) (Lithium Iodide) Reaction with Acids Lithium reacts slowly with water, and forming a colorless solution of Lithium hydroxide (LiOH) and hydrogen gas (H 2).Ģ Li (s) + 2 H 2O (l) → 2 LiOH (aq) + H 2 (g) Reaction with Halogens If burned the metal, a minor amount of Peroxide (Li 2O 2) is produces as well.Ģ Li (s) + O 2 (g) → Li 2O 2 (s) Reaction with Water The metal reacts slowly with Oxygen (O 2) at normal temperature, and forming Lithium oxide (Li 2O). Ionization energies: 1st: 520.2 kJ.mol 2nd: 7298.1 kJ/mol 3rd: 11815 kJ/molīody Centered Cubic (BCC) Reactivity of LithiumĮlectron affinity: 59.5 kJ/mol Nuclear Properties of LithiumĬhemical Reactions of Lithium Element Reaction with Air The ionization potential of an atom: 5.37 eV Sound Speed: 6000 m/s Atomic Properties of Lithium Molar magnetic susceptibility: 0.178×10 -9 m 3/mol Physical Properties of Lithiumĭensity: 0.534 g/cm 3 (In solid) 0.512 g/cm 3 (In Liquid at M.P) Mass magnetic susceptibility: 6.3×10 -9 m 3/kg Volume magnetic susceptibility: 0.000000337 Magnetic susceptibility (x mol): +14.2×10 -6 cm 3/mol Neel Point (magnetic ordering temperature) T N: N/A Electrical properties of LithiumĬritical point (Superconducting point): N/A Magnetic Properties of Lithium Lithium Electron Configuration Thermal Properties of Lithium So it should be stored in a Non-reactive liquid hydrocarbon such as Naphtha (flammable liquid hydrocarbon mixture). Metallic Lithium is soluble in short chain aliphatic amines, like Ethylamine (C 2H 5NH 2), but insoluble in hydrocarbons.

The element Reacts violently with strong oxidants, acids and many compounds (halons, halogens, hydrocarbons, concrete, sand and asbestos) causing fire and explosion hazard. The element surface becomes coated with a mixture of Lithium hydroxide (LiOH), Lithium nitride (Li 3N), and Lithium carbonate (Li 2CO 3).

When the Lithium placed over a flame, this metal gives off a beautiful crimson color but when it burns strongly, the flame becomes a dazziling white. While reacts with water It forms highly flammable hydrogen gas and corrosive fumes of Lithium hydroxide (LiOH). It reacts vigorously with water, but not as vigorously as sodium. Like the other alkali metals, Pure Lithium element is highly flammable and slightly explosive when exposed to air and especially water. It has the lowest density of all metals, which makes it the Lighest soild element. Pure Lithium element is a silver white, soft material. Bibliographic Databases on Atomic Spectroscopy.


 0 kommentar(er)
0 kommentar(er)
